A global reptile assessment highlights shared conservation needs of tetrapods - Nature.com
Abstract
Comprehensive assessments of species' extinction risks have documented the extinction crisis1 and underpinned strategies for reducing those risks2. Global assessments reveal that, among tetrapods, 40.7% of amphibians, 25.4% of mammals and 13.6% of birds are threatened with extinction3. Because global assessments have been lacking, reptiles have been omitted from conservation-prioritization analyses that encompass other tetrapods4,5,6,7. Reptiles are unusually diverse in arid regions, suggesting that they may have different conservation needs6. Here we provide a comprehensive extinction-risk assessment of reptiles and show that at least 1,829 out of 10,196 species (21.1%) are threatened—confirming a previous extrapolation8 and representing 15.6 billion years of phylogenetic diversity. Reptiles are threatened by the same major factors that threaten other tetrapods—agriculture, logging, urban development and invasive species—although the threat posed by climate change remains uncertain. Reptiles inhabiting forests, where these threats are strongest, are more threatened than those in arid habitats, contrary to our prediction. Birds, mammals and amphibians are unexpectedly good surrogates for the conservation of reptiles, although threatened reptiles with the smallest ranges tend to be isolated from other threatened tetrapods. Although some reptiles—including most species of crocodiles and turtles—require urgent, targeted action to prevent extinctions, efforts to protect other tetrapods, such as habitat preservation and control of trade and invasive species, will probably also benefit many reptiles.
Main
Although comprehensive extinction-risk assessments have been available for birds, mammals and amphibians for well over a decade3, reptiles have, until now, not been comprehensively assessed. Therefore, conservation science and practice has typically relied on the International Union for Conservation of Nature (IUCN) Red List categories and distributions of the other three tetrapod classes to inform policy and guide priorities for investments2, despite differing expectations as to how effective common strategies will be across classes9,10. With a high diversity in arid regions and some islands and archipelagos (for example, Antilles, New Caledonia and New Zealand) compared with other tetrapods, reptiles were thought to require different conservation strategies and geographical priorities6. In the absence of Red List assessments, researchers have resorted to indirect measures of extinction risk such as range size and human pressure6,11. Here we examine the results of a comprehensive Red List assessment of reptiles and outline their implications for the conservation needs of reptiles.
Comprising the turtles (Testudines), crocodiles (Crocodylia), squamates (Squamata: lizards, snakes and amphisbaenians) and tuatara (Rhynchocephalia), reptiles are a paraphyletic class representing diverse body forms, habitat affinities and functional roles in their respective ecosystems12. The largely terrestrial squamates are by far the most speciose group (9,820 species in this assessment), whereas the primarily aquatic turtles and crocodiles are often larger bodied but include only 351 and 24 species, respectively. Rhynchocephalians diverged from the snake and lizard lineage in the Triassic period and include one extant species13. Given this diversity of reptiles, threats to their persistence are likely to be equally varied, and so these need to be specified to guide effective conservation action.
Extinction risk and threats
We assessed reptiles globally using the IUCN Red List criteria with input from 961 scientists (Supplementary Note 1) achieved primarily through 48 workshops (Supplementary Table 1). Across all 10,196 species assessed, 21.1% are threatened with extinction (categorized as vulnerable, endangered or critically endangered; Supplementary Table 2). As a group, a greater number of reptile species are threatened than birds or mammals, but fewer than amphibians. Proportionately more mammals and amphibians are threatened than reptiles (Fig. 1a). The reptile threat prevalence falls within a previous estimate of 15–36% threatened (best estimate 19%) from a random sample of 1,500 reptile species8. To our knowledge, this study represents the first global test of a sampled Red List extrapolation. The proportion of turtles and crocodiles that are threatened (57.9% and 50.0%, respectively) is much higher than those of squamates (19.6%) and tuatara (0%), and comparable to the most-threatened tetrapod groups, salamanders (57.0%) and monotremes (60.0%) (Fig. 1b). Within squamates, iguanid (73.8%) and xenosaurid (60.0%) lizards and uropeltid (61.1%) and tropidophiid (60.0%) snakes are highly threatened. Since 1500, 31 reptile species (0.3%) have been driven extinct, including 24 squamates and 7 turtles, with 2 squamate species from Christmas Island categorized as extinct in the wild (persisting only as captive populations); 40 critically endangered species are 'possibly extinct' (that is, species that are likely to be extinct, but that have a small chance that they may be extant; Extended Data Fig. 1). Additional species probably became extinct before being documented by science14.
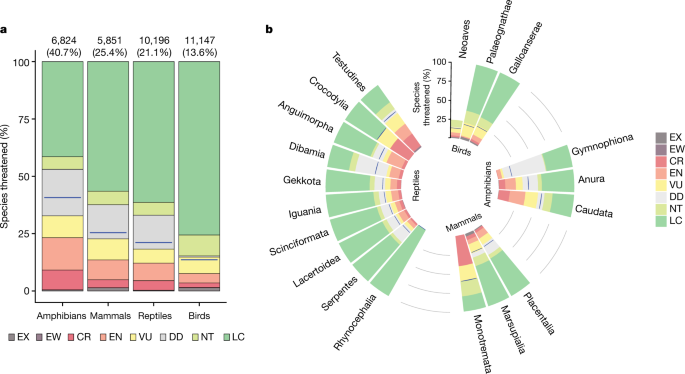
a, Taxonomic patterns organized by class. The numbers above each column refer to the numbers and percentages of species threatened (that is, those categorized as critically endangered, endangered or vulnerable). b, Extinction risk by major taxonomic groups. Blue lines indicate the best estimate of the percentage of species threatened. CR, critically endangered; DD, data deficient; EN, endangered; EW, extinct in the wild; EX, extinct; LC, least concern; NT, near threatened; VU, vulnerable.
Source data
Limited information resulted in 1,507 species (14.8%) being categorized as data deficient, similar to the number in mammals (15.1%) and lower than for amphibians (20.4%), but much higher than for birds (0.5%). Taxonomic groups with fossorial or other secretive habits and/or restricted to poorly studied areas (such as blindsnakes (Gerrhopilidae and Typhlopidae)) had greater proportions of species categorized as data deficient (Supplementary Table 2). The greatest numbers of data-deficient species occur in Asia (585), South America (284) and Africa (271), with fewer data-deficient species in North and Middle America (163), Oceania (124), Australia (55), the Caribbean (34) and Europe (3). Uncertainty about the status of data-deficient species suggests that the proportion of reptiles threatened with extinction ranges from 18.0% (assuming no data-deficient species are threatened) to 32.8% (assuming all data-deficient species are threatened) with a best estimate of 21.1%.
Concentrations of threatened reptiles are mostly in regions in which other tetrapods are also threatened (Extended Data Fig. 2). Threatened reptiles are concentrated in southeastern Asia, West Africa, northern Madagascar, the northern Andes and the Caribbean, but largely absent from Australian drylands; the Kalahari, Karoo and Sahara deserts; northern Eurasia; and the Rocky Mountains and northern North America (Fig. 2a). In remarkably few regions, however, are reptiles disproportionately threatened relative to other tetrapods (that is, have at least twice the number of species in a threatened category): parts of southern Asia and northeastern USA (Fig. 2b and Extended Data Table 1). Moreover, for most (87%) terrestrial regions in which tetrapods occur, no tetrapod class is disproportionately threatened compared with the other classes.
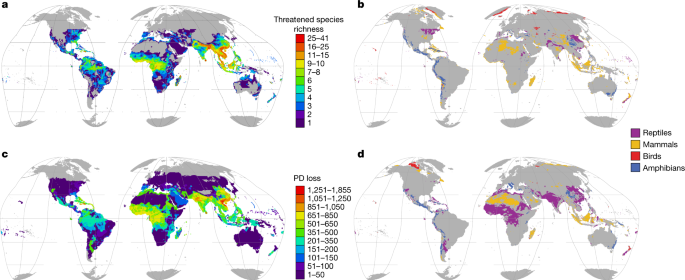
a, Distribution of reptile species that are threatened (critically endangered, endangered or vulnerable). b, Regions with disproportionate numbers of threatened species for each tetrapod class (areas for each class where the proportional threat in species diversity is at least twice the loss for the next-most threatened class). c, Loss of reptile phylogenetic diversity (PD) if all threatened species became extinct. d, Regions with disproportionate phylogenetic diversity loss for each tetrapod class (calculated as in b). Grey, areas with no threatened species (a, c) or regions in which no class is disproportionately threatened (b, d). Data are shown at a resolution of 50 km.
With deep phylogenetic lineages and high species diversity, reptiles stand to lose a large amount of phylogenetic diversity (a measure of difference within an evolutionary tree15) if the current extinction crisis continues apace. Assuming all threatened species (and only these species) become extinct, the combined loss of reptile phylogenetic diversity (calculated using existing phylogenetic trees16,17) will be approximately 15.6 billion years. Southeastern Asia, India, West Africa and the Caribbean8 (Fig. 2c) comprise the top 15% areas of phylogenetic diversity loss, with high concentrations of threatened and evolutionarily distinct species (Extended Data Fig. 3). Comparing the distributions of threatened phylogenetic diversity across all four tetrapod groups reveal relatively small geographical areas of disproportionate importance for any class (Fig. 2d and Extended Data Table 2).
The anthropogenic factors increasing extinction risk in reptiles are mainly habitat destruction from agricultural expansion, urban development and logging (Fig. 3a). Other important threats are invasive species and hunting, which includes commercial harvest and trade (Fig. 3a). Among reptile groups, crocodiles and turtles are most frequently affected by hunting and less by agriculture, whereas squamates are most frequently threatened by agriculture (Fig. 3a). The major threats are broadly similar across tetrapods (Fig. 3b). For all tetrapod groups, agriculture threatens the most species, logging is the second or third most prevalent threat, and invasive species and disease are the fourth or fifth most prevalent threat. Threats causing habitat destruction (complete removal of habitat) affect proportionately more species than those causing habitat change (degradation of habitat). The largest differences in relative threat prevalence are for hunting, which threatens mammals much more than the other tetrapods, and urban development, which affects amphibians, reptiles and mammals more than birds.
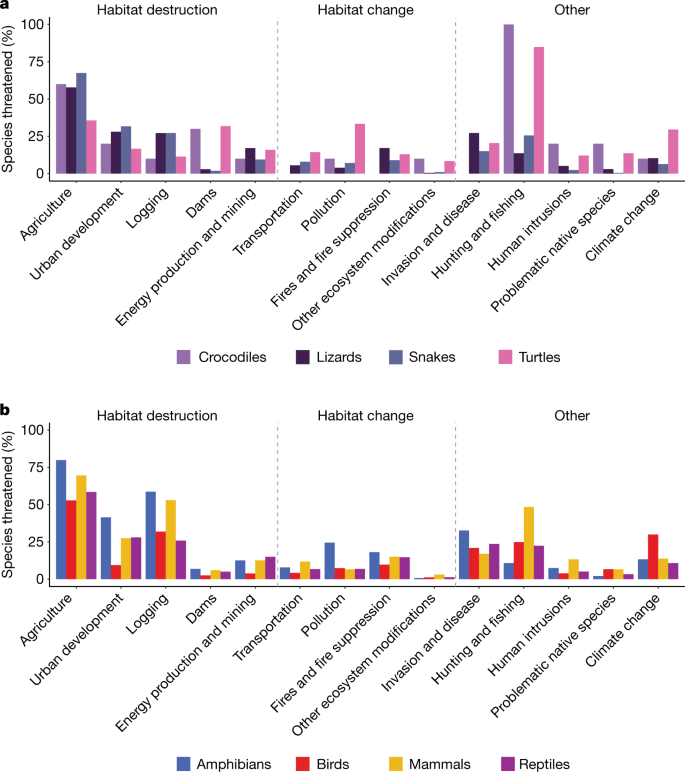
a, Crocodiles, lizards (including amphisbaenians), snakes and turtles. b, All tetrapods. Only threats to species categorized as critically endangered, endangered or vulnerable were included. Some species are subject to more than one threat (mean = 2.4; s.d. = 1.3 threats per species).
Source data
Climate change is a looming threat to reptiles, for example, by reducing thermally viable windows for foraging18, skewing offspring sex ratios in species that have temperature-dependent sex determination19 and contracting ranges20. Given the Red List three-generation horizon for assessments, the lack of long-term studies limits the documentation of climate change as a near-future threat to reptiles21, in contrast to, for example, birds (Fig. 3b). Disease is documented as a threat for only 11 species of reptiles (<1% of extant, non-data-deficient species), although pathogens such as Ophidiomyces ophiodiicola (which causes snake fungal disease22) pose a potential threat and are little studied outside North America. Intentional use of reptiles (local consumption and trade) is an important threat to reptiles23, and was found to threaten 329 species (3.2%), especially turtles (30.8% of all turtle species).
More than half of all reptile species occur in forested habitats (Fig. 4c). Although some reptiles, particularly lizards, are speciose in arid or seasonally dry habitats such as deserts, grasslands, shrublands and savannahs6,24, these species are less threatened than those occupying forest habitats (13.7% of species restricted to arid habitats versus 26.6% of species restricted to forests; Fisher's exact test, P = 0.00001; Fig. 4b). The top threats to reptiles—agriculture, urban development and logging—are also the top threats to species inhabiting forested habitats, affecting 65.9%, 34.8% and 27.9% of forest-dwelling threatened reptiles, respectively, helping to explain the higher extinction risk of forest species. Agriculture and logging are significantly more likely to threaten forest-dwelling than non-forest dwelling reptiles (Fisher's exact test, P = 0.00001), whereas urbanization threatens forest-dwelling no differently than non-forest dwelling reptiles (Fisher's exact test, P = 0.25). Turtles and crocodiles are much more frequently associated with wetlands than other reptiles (Fig. 4a).
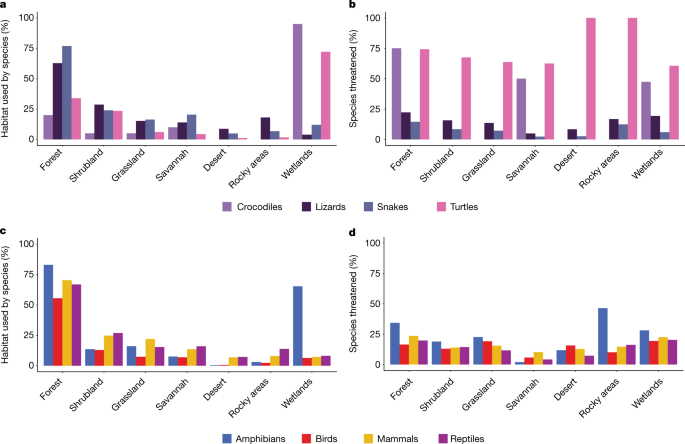
a, Habitats used by crocodiles, lizards (includes amphisbaenians), snakes and turtles. b, Percentage of reptiles using each habitat that are threatened. c, Habitats used by tetrapods. d, Percentage of threatened tetrapod species using each habitat. See Supplementary Table 3 for additional, rarely used habitats not shown here. Artificial habitats are not shown.
Source data
Like reptiles, more than twice as many bird and mammal species occur in forests compared with any other habitat type (Fig. 4c). Forests are also the most common habitat for amphibians, although wetlands are important for many species, especially for breeding (Fig. 4c). Also similar to reptiles, the proportions of forest-inhabiting bird, mammal and amphibian species that are threatened are higher than for species that do not inhabit forests (16.7% versus 13.0%, 27.5% versus 20%, and 42.4% versus 34.4%, respectively; Fisher's exact tests, P = 0.00001). Threat levels for each tetrapod class in arid habitats tend to be lower (less than 23% of species occurring in such regions). Across tetrapods, forests support high diversity and are also subject to widespread threats.
Surrogacy of other tetrapods for reptiles
With numerous threatened tetrapod species (227 birds, 194 mammals, 607 amphibians and 474 reptiles) ranging completely outside formally protected areas, assessing surrogacy is important to gauge the magnitude of efforts needed to conserve these species. We addressed surrogacy using a complementarity representation approach for threatened species, which better addresses the extent to which areas selected for surrogates capture target features than, for example, spatial congruence25. When combined, threatened birds, mammals and amphibians—the tetrapod groups for which comprehensive Red List data were previously available—are good surrogates for the conservation of threatened reptile diversity when prioritizing the richness of rarity-weighted threatened species at both 50-km and 100-km resolution (median species accumulation indices are 0.66 and 0.76, respectively; Extended Data Fig. 4a). Using this same prioritization strategy, birds and mammals individually are reasonable surrogates for reptiles, whereas amphibians are poor surrogates (Extended Data Fig. 4a). By contrast, for a complementarity representation strategy that prioritizes individual threatened species with the smallest ranges, birds, mammals and amphibians are not good surrogates for reptiles (species accumulation indices < 0.40), although combined they are reasonable surrogates at both 50-km and 100-km resolutions (median species accumulation indices are 0.44 and 0.64, respectively; Extended Data Fig. 4b). These results indicate that the smallest-ranged threatened reptiles tend to be isolated from threatened birds, mammals and amphibians. In addition, priority areas for threatened birds and mammals independently, and birds, mammals and amphibians combined, showed high spatial congruence with priority sites for threatened reptiles for both strategies (prioritizing either rarity-weighted threatened species or complementarity representation), although correlations among global portfolios of priority areas were lower (Extended Data Tables 3, 4). Although our results for the smallest ranged threatened species are consistent with previous expectations of low surrogacy9, overall, we found reasonably high surrogacy10.
Discussion
Our discovery of broad similarities in the geography and nature of threats between reptiles and other tetrapods was unexpected given previous arguments about the exceptionalism of reptiles for being particularly diverse in arid habitats6. The implications for tetrapod conservation are that geographical prioritizations previously performed for birds, mammals and amphibians overlap broadly with prioritizations for all except the most range-restricted threatened reptiles. The absence of reptiles in many global conservation prioritization analyses to date is unlikely to have left the class less represented than others. Nevertheless, the low surrogacy value of other tetrapods for reptiles with the most restricted ranges suggests that a case-by-case focus is required for these microendemics. Indeed, the ranges of 31 threatened reptiles do not overlap with the ranges of any other threatened tetrapod (among threatened species, 84 birds, 11 mammals and 7 amphibians are similarly isolated from other threatened tetrapods) (Supplementary Table 3).
Researchers have predicted that reptiles are particularly vulnerable to climate change in tropical biomes26 as well as freshwater27 and arid habitats18, although so far no clear geographical signal in reptile declines due to climate change has been detected28. If such vulnerabilities are found, then—as climate change continues to alter the distributions and extinction risk of species—the surrogacy across tetrapods could unravel with, for example, reptiles in specific habitat types declining swiftly and disproportionately (relative to other tetrapods).
Among the conservation strategies needed to prevent reptile extinction, land protection is critically important to buffer many threatened species from the dual threats of agricultural activities and urban development. The hundreds of threatened reptiles that currently occur completely outside protected areas underscore the need for targeted safeguards of important sites. Beyond place-based strategies, conservation policy and practice must halt unsustainable harvest and stem the spread of invasive disease to prevent many more species from becoming threatened23. Furthermore, introduced mammals to islands threaten 257 reptile species (2.8% of all reptiles), calling for continued campaigns to eradicate introduced mammals in those places.
With a comprehensive, global assessment of the extinction risk of reptile species now available, these data can be incorporated into the toolbox of conservation practice and policy. At the species level, they can serve as the starting point for 'green status' (formerly 'green list') assessments that define, measure and incentivize species recovery29. More generally, they can be integrated into the calculation of species threat abatement and restoration metrics2, the identification of key biodiversity areas30 and resource allocation using systematic conservation planning31, all of which have primarily been dependent on data from birds, mammals and amphibians among animals to date. Future reassessments will allow reptile data to be included in the Red List Index32, a widely used indicator of biodiversity trends1.
Although efforts aimed at protecting other threatened tetrapods probably benefit many of the 1,829 threatened reptiles—especially forest-dwelling species—conservation investments targeted at uniquely occurring reptiles or those requiring tailored policies must also be implemented to prevent extinction. Encouragingly, the First Draft of the Post-2020 Global Biodiversity Framework33 to be agreed by governments in 2022 explicitly targets safeguarding important sites (target 3), complemented by emergency action for individual threatened species (target 4). This political determination to reverse the slide of species toward extinction bodes well for reptiles.
Methods
We used the IUCN Red List criteria34,35 and methods developed in other global status-assessment efforts36,37 to assess 10,078 reptile species for extinction risk. We additionally include recommended Red List categories for 118 turtle species38, for a total of 10,196 species covered, representing 89% of the 11,341 described reptile species as of August 202039.
Data compilation
We compiled assessment data primarily through regional in-person and remote (that is, through phone and email) workshops with species experts (9,536 species) and consultation with IUCN Species Survival Commission Specialist Groups and stand-alone Red List Authorities (442 species, primarily marine turtles, terrestrial and freshwater turtles, iguanas, sea snakes, mainland African chameleons and crocodiles). We conducted 48 workshops between 2004 and 2019 (Supplementary Table 1). Workshop participants provided information to complete the required species assessment fields (geographical distribution, population abundance and trends, habitat and ecological requirements, threats, use and trade, literature) and draw a distribution map. We then applied the Red List criteria34 to this information to assign a Red List category: extinct, extinct in the wild, critically endangered, endangered, vulnerable, near threatened, least concern and data deficient. Threatened species are those categorized as critically endangered, endangered and vulnerable.
Taxonomy
We used The Reptile Database39 as a taxonomic standard, diverging only to follow well-justified taxonomic standards from the IUCN Species Survival Commission40. We could not revisit new descriptions for most regions after the end of the original assessment, so the final species list is not fully consistent with any single release of The Reptile Database.
Distribution maps
Where data allowed, we developed distribution maps in Esri shapefile format using the IUCN mapping guidelines41 (1,003 species). These maps are typically broad polygons that encompass all known localities, with provisions made to show obvious discontinuity in areas of unsuitable habitat. Each polygon is coded according to species' presence (extant, possibly extant or extinct) and origin (native, introduced or reintroduced)41. For some regions covered in workshops (Caucasus, Southeast Asia, much of Africa, Australia and western South America), we collaborated with the Global Assessment of Reptile Distributions (GARD) (http://www.gardinitiative.org/) to provide contributing experts with a baseline species distribution map for review. Although refined maps were returned to the GARD team, not all of these maps have been incorporated into the GARD.
Habitat preferences
Where known, species habitats were coded using the IUCN Habitat Classification Scheme (v.3.1) (https://www.iucnredlist.org/resources/habitat-classification-scheme). Species were assigned to all habitat classes in which they are known to occur. Where possible, habitat suitability (suitable, marginal or unknown) and major importance (yes or no) was recorded. Habitat data were available for 9,484 reptile species.
Threats
All known historical, current and projected (within 10 years or 3 generations, whichever is the longest; generation time estimated, when not available, from related species for which it is known; generation time recorded for 76.3% of the 186 species categorized as threatened under Red List criteria A and C1, the only criteria using generation length) threats were coded using the IUCN Threats Classification Scheme v.3.2 (https://www.iucnredlist.org/resources/threat-classification-scheme), which follows a previously published study42. Where possible, the scope (whole (>90%), majority (50–90%), minority (<50%) of the population; unknown) and severity (causing very rapid (>30%), rapid (>20%), slow but notable (<20%) declines over 10 years or 3 generations, whichever is longer; negligible declines; unknown) of the threat was recorded. Threat data were available for 1,756 of the 1,829 threatened reptile species.
Assessment review
Each assessment underwent two reviews. First, a scientist familiar with the species but not involved in the assessment reviewed the account for biological accuracy and accurate application of the Red List criteria. Once the assessors revised the assessment satisfactorily, staff from the IUCN Red List Unit reviewed the assessment primarily for accurate application of the Red List criteria. The assessors revised the assessment again, if necessary, to satisfy any concerns of the IUCN Red List Unit before the assessment was finalized.
Data limitations
Although we made an extensive effort to complete assessments for all reptiles, some data gaps remain.
Missing species
As of December 2020, 1,145 reptile species, primarily snakes and lizards, were omitted from the present study, including the phylogenetic diversity analyses, because they were described recently and they were described after previous comprehensive assessments from the region. Geographically, they are primarily from tropical regions (as are assessed reptiles) with an underrepresentation of African species (distribution of omitted species: Asia, 41%; Africa, 8%; Australia, 7%; Europe, 3%; North/Central America, 20%; South America, 19%; Caribbean, 5%; Oceania, 4%; percentages sum to greater than 100% because some species occur in two regions). Because they are recently described, many are poorly known, may be rare or occur in a very restricted area, or in poorly surveyed areas that are often subject to high levels of human impacts. As such, recent descriptions are more likely to receive a data-deficient or threatened Red List category than be assigned of least concern41. The net effect on our analyses is a slight underestimate of the number of threatened snakes and lizards, and plausibly a slight overestimate of least concern species. With tetrapod species described in the future likely to be small-ranged, threatened lizards and amphibians43,44, surrogacy levels may decline from those reported here.
Geographical coverage
Although we made extensive efforts to map the current known distribution for each species, this information is incomplete for some species. Where appropriate, and following expert guidance, we interpolated between known localities if the ecological conditions appeared appropriate. In addition, species occurrence is unlikely to be spread evenly or entirely throughout the area depicted in range maps, with gaps expected, for example, in patches of unsuitable habitat.
Data-deficient species
For species assessed to be data deficient (1,507 reptiles, 14.8%), there was inadequate information on the distribution, population status or threats (historical, current or projected future) of the species (both from published sources and expert knowledge) to make a direct, or indirect, assessment of the risk of extinction. All species were assessed according to their recognized taxonomic circumscription at the time of assessment. Taxonomic uncertainty therefore did not result in a data-deficient assignment, although some species were listed as data deficient because they are morphologically indistinguishable from another species and therefore estimates of distribution and abundance are not feasible.
Time span of assessments
The assessments were completed between 1996 and 2020, with 1,503 assessments completed before 2011. The IUCN Rules of Procedure (https://www.iucnredlist.org/resources/rules-of-procedure) recommend reassessment every 10 years and thus, as of 2020, 15% of the assessments can be considered outdated. Of the species assessed 1996–2010, slightly more species were threatened (23.0%) than the species assessed more recently (20.7%). This difference is largely explained by the greater percentages of crocodiles and turtles with outdated assessments (29% and 35%, respectively) compared with tuatara, lizards and snakes (0%, 12% and 17%, respectively) and the highly threatened nature of crocodiles and turtles (Supplementary Table 2). The continuing deterioration of biodiversity globally1 suggests that the species with outdated assessments are more likely to be in higher threat categories today than when they were when last assessed, causing an underestimation of current reptile threat status.
Analyses
Percentage of species threatened with extinction
To estimate the percentage of species threatened with extinction (categories critically endangered, endangered and vulnerable), we used the following formula, which assumes that data-deficient species have the same proportion of threatened species as species that were not data deficient.
where Propthreat is the best estimate of the proportion of species that are threatened; CR, EN, VU and DD are the number of species in each corresponding Red List category and N is the number of species assessed (excluding extinct and extinct in the wild species).
Data for amphibians, birds and mammals
For all analyses that included data for amphibians, birds and mammals, we used the 2020-1 version3 of the tabular and spatial data downloaded from the IUCN Red List website in May 2020.
Threats
Threats calculations were restricted to species in threatened Red List categories (critically endangered, endangered and vulnerable). Multiple threats can affect a single species. Summaries of threats are for the first level of the IUCN classification scheme. Threats thought to affect only a minority of the global population (<50% of the population) (coded as 'minority') were not included. In addition, we removed threats that were assessed to cause 'no declines' and 'negligible declines' from the analysis (as indicated by the severity coding). We considered all threats without scope or severity scored to be major threats and retained them in the analysis.
Habitat
Analyses of habitat use were restricted to the first level of the IUCN habitat-classification scheme. We excluded habitats for which the major importance to the species was scored 'no' and suitability was scored 'marginal' and considered all habitats without major importance or suitability scored to be suitable and of major importance and included them in the analyses. We did not consider artificial habitats in the analyses.
Only a small number of reptile species inhabits 'caves/subterranean' and 'marine coastal' habitats, so they were not included in Fig. 4 but their threat prevalence is summarized in Supplementary Table 4.
Statistics
Statistical tests were designed to avoid inclusion of multiple observations from the same species (because species can occur in multiple habitats and be threatened by multiple threats). To assess whether arid habitat or forest species were more likely to be threatened, we included only species that were restricted to one of these habitat types. For threats analyses, we compared species that occur in forests (including those that occur in forests and other habitats) to those that do not occur in forests. All tests were two-tailed Fisher's exact tests.
Geographical patterns
The geographical patterns of threat and phylogenetic diversity shown in Fig. 2 are for only terrestrial species (so, for reptiles, excluding 87 species of marine turtles and sea snakes). Tetrapod classes vary widely in the numbers of pelagic marine species and in the methods used to map distributions. Restricting analyses to terrestrial species ensured more-consistent analyses and avoided wide variation in summary values caused by small numbers of species.
Analyses of the distribution maps used polygons either with the following IUCN map code designations or with no codes indicated:
Presence = extant (code 1) and probably extant (code 2)
Origin = native (code 1), reintroduced (code 2) and introduced (code 6)
Seasonality = resident (code 1), breeding season (code 2), non-breeding season (code 3) and passage (code 4).
Ranges for species categorized as critically endangered (possibly extinct) are coded as possibly extinct (code 4) and excluded from the spatial analyses.
All spatial analyses were conducted on a global 0.5° by 0.5 ° latitude–longitude grid (approximately 50 km at the Equator). To explore the influence of spatial resolution, we repeated the surrogacy and phylogenetic diversity analyses at a 100-km resolution. We converted polygon range maps (tagged with the appropriate codes as described above) to these grids. We used a global equal-area pseudocylindrical projection, Goode homolosine.
We mapped the distribution of threatened species as a count of the number of species with ranges overlapping each grid cell.
Estimating the spatial distribution of disproportionate threat and phylogenetic diversity loss
We identified global areas in which each tetrapod class is disproportionately threatened compared with all other classes by comparing the species-richness-adjusted level of threat among the four tetrapod classes. First, for each grid cell, we identified the proportional threat level of each class by dividing the number of species in threatened Red List categories (vulnerable, endangered and critically endangered) by the total number of species for the class found in that cell. Second, for all grid cells in which at least five tetrapod species are present, we compared proportional threat values across the four classes and identified a grid cell as having a disproportionate threat level for a given class if: (1) the grid cell had a proportional level of threat equal to 10% or higher for the class; and (2) the grid cell had a proportional level of threat for the class at least twice as high as the proportional level of threat for the next class. We assessed the sensitivity of disproportionate threat patterns to our definition of disproportionate threat by varying the degree of difference in proportional threat level between the highest and second highest class. We identified the number of grid cells with disproportionate threat for each class when the class had a proportional threat level: (1) higher than any other class; (2) 25% or more higher than any other class; (3) 50% or more higher than any other class; (4) 100% or more higher than any other class; and (5) 200% or more higher than any other class. In the main text, we report results for the 100% or more threat level. Results for all thresholds are included in Extended Data Tables 1, 2.
Conservation strategies
We identified global conservation priorities for each tetrapod class using two alternative strategies: strategy 1 prioritized areas containing many threatened species with relatively highly restricted ranges, whereas strategy 2 prioritized areas core to the most range-restricted threatened species globally. We implemented both conservation strategies within the spatial conservation-planning software Zonation45 and the R package zonator46, using the additive benefit function and the core-area Zonation algorithms for strategies 1 and 2, respectively, at 50-km and 100-km resolutions for threatened reptiles.
The additive benefit function algorithm prioritizes areas by the sum of the proportion of the global range size of all species included in a given grid cell—a quantity similar to weighted species endemism (as defined previously47) and endemism richness (as defined48). On the basis of this algorithm, cells with many species occurring only in that cell or few other cells receive the highest priority. The core-area Zonation algorithm prioritizes areas by the maximum proportion of the global range size of all species included in a given grid cell: cells including the highest proportions of the ranges of the most range-restricted species are given the highest priority.
Therefore, comparing the two strategies, strategy 1 gives more importance to the number of species within grid cells (that is, more species = a higher summed proportion), potentially at the expense of the single most-range-restricted species globally, which are instead prioritized directly by strategy 2.
Because complementary representation problems such as these spatial prioritizations often have multiple solutions, we ran five iterations of each algorithm used and summarized variation across those.
Estimating surrogacy
To assess the degree to which conserving the diversity of threatened species of birds, mammals and amphibians (individually or combined) serves as a surrogate for conserving threatened reptile diversity, we calculated a species accumulation index (SAI) of surrogate effectiveness. The SAI is derived from the comparison of three curves: (1) the 'optimal curve' represents the accumulation of the diversity of threatened reptile species when conservation is planned using data for threatened reptiles directly; (2) the 'surrogacy curve' represents the accumulation of the diversity of threatened reptile species when conservation is planned using the diversity of threatened species diversity of a different class as a surrogate; and (3) the 'random curve' represents the accumulation of the diversity of threatened reptile species when conservation areas are selected at random. We estimated optimal, surrogate and random curves based on each reptile-surrogate combination (birds, mammals and amphibians individually and combined). Using 100 sets of approximately random terrestrial grid-cell sequences allowed us to generate 95% confidence intervals around a median 'random curve'. In addition, because we ran five iterations of each spatial prioritization algorithm for each tetrapod class, optimal and surrogate curves were also summarized using the median and 95% confidence intervals across the five iterations.
We then derived the quantitative measure of surrogacy as SAI = (s − r)/(o − r), where s is the area under the surrogate curve, r is the area under the random curve and o is the area under the optimal curve. SAI = 1 when the optimal and surrogate curves are the same (perfect surrogacy). It is between 1 and 0 when the surrogate curve lies above the random curve (positive surrogacy), zero when the surrogate and random curves coincide (no surrogacy) and negative when the surrogate curve lies below the random curve (negative surrogacy). We calculated the SAI using R code modified from a previous study49. For each reptile–surrogate combination, we report median and 95% confidence intervals across all combinations of optimal, surrogate and random curves (5 target and surrogate curve iterations and 100 random curve iterations).
Although not strictly a measure of surrogacy25, we also calculated the spatial congruence (Spearman's rank correlation, analogous to a previously published approach9) of Zonation priorities for each conservation strategy and spatial resolution.
Coverage by protected areas
We overlayed protected areas (polygons, categorized as IUCN I–VI from the World Database of Protected Areas50) over the ranges of all threatened tetrapods and classified species with ranges completely outside any protected area as unprotected.
Phylogenetic diversity
To calculate phylogenetic diversity15, we used published time trees of mammals51, birds52 and amphibians53. For reptiles, we combined two time trees: a comprehensive squamate time tree containing 9,755 squamate species, including the species Sphenodon punctatus16, and a turtle and crocodilian tree containing 384 species17. The time trees contain some species lacking genetic data, added by taxonomic interpolation54 to maximize taxonomic coverage. In total, we analysed 32,722 tetrapod species including 10,139 reptiles, 5,364 mammals, 9,879 birds and 7,239 amphibians. For squamates, and for turtles and crocodiles, 10,000 fully resolved trees were available. For each group,...

Comments
Post a Comment