Exceptional parallelisms characterize the evolutionary transition to live birth in phrynosomatid lizards - Nature.com
Abstract
Viviparity, an innovation enhancing maternal control over developing embryos, has evolved >150 times in vertebrates, and has been proposed as an adaptation to inhabit cold habitats. Yet, the behavioral, physiological, morphological, and life history features associated with live-bearing remain unclear. Here, we capitalize on repeated origins of viviparity in phrynosomatid lizards to tease apart the phenotypic patterns associated with this innovation. Using data from 125 species and phylogenetic approaches, we find that viviparous phrynosomatids repeatedly evolved a more cool-adjusted thermal physiology than their oviparous relatives. Through precise thermoregulatory behavior viviparous phrynosomatids are cool-adjusted even in warm environments, and oviparous phrynosomatids warm-adjusted even in cool environments. Convergent behavioral shifts in viviparous species reduce energetic demand during activity, which may help offset the costs of protracted gestation. Whereas dam and offspring body size are similar among both parity modes, annual fecundity repeatedly decreases in viviparous lineages. Thus, viviparity is associated with a lower energetic allocation into production. Together, our results indicate that oviparity and viviparity are on opposing ends of the fast-slow life history continuum in both warm and cool environments. In this sense, the 'cold climate hypothesis' fits into a broader range of energetic/life history trade-offs that influence transitions to viviparity.
Introduction
Live-bearing (viviparity) is a major evolutionary novelty in the tree of life that affords physiological control and protection to developing embryos, providing higher offspring survivorship1,2,3,4,5. There are at least 150 independent origins of viviparity in vertebrates, particularly in squamate reptiles (>115 origins)6,7. Given their strong representation in relatively cool habitats, live-bearing in squamates has been classically interpreted as an adaption to lower environmental temperatures (i.e., the 'cold-climate hypothesis', or CCH): relative to eggs deposited in nests, incubation within the dam provides a relatively stable (and warmer) temperature, in turn shortening developmental time and enhancing offspring viability in cool environments3,8,9. Complete embryogenesis within the female reproductive tract may also be accompanied by adjustments in life history, morphology, thermoregulatory behavior, and thermal physiology10,11,12,13. Although the transition from egg laying to live-bearing has repeatedly arisen, whether phenotypic evolution in viviparous lineages is also repeatable remains unclear. For example, to what extent are phenotypic shifts correlated or decoupled among transitions to live birth? And, how strongly do phenotypic patterns among different viviparous lineages relate to the thermal environment and to the cold-climate hypothesis?
One key lens with which to approach these questions is by examining the ratio by which energy is acquired and allocated to survival, growth, and reproduction (metabolic rate), and the energy allocated to the number and size of hatchlings (production). Compared to oviparous counterparts, viviparous species often exhibit lower mass-specific metabolic rates14,15 and reduced mass-specific production16. Nonetheless, it remains unclear whether mass-specific metabolic rates and mass-specific production shift in tandem or independently of each other in viviparous lineages. Metabolic rate increases with body mass and body temperature (see equation in Methods)17,18. Shifts in morphology and thermal physiology, therefore, can produce several trait combinations that result in a lower mass-specific metabolic rate18 in viviparous species (Table 1a). In another way, mass-specific production is determined by the trade-off between offspring mass and the number of hatchlings or eggs produced yearly (annual fecundity) divided by female body mass16,19. Thus, the lower mass-specific production of viviparous species may reflect different combinations of trait shifts (Table 1b). What combinations between body mass and body temperature are associated with patterns of mass-specific metabolic rate in viviparous linages? Likewise, are shifts in offspring mass and/or offspring number (annual fecundity) evolving in tandem or independently among viviparous lineages? More broadly, how does thermal physiology (beyond just field body temperatures for activity) evolve in viviparous lineages and, in light of the CCH, how strongly do shifts in the thermal environment predict parity mode evolution?
The repeated origin of viviparity among closely-related species provides a naturally replicated framework in which to test for shared signatures of adaptation. Squamate reptiles (lizards and snakes) account for ~75% of the origins of viviparous vertebrates7. Here, we focused on phrynosomatid lizards, a lineage well known for repeated transitions to live birth20,21, to investigate the associations between behavior, physiology, morphology, and life history associated with viviparity. This diverse lizard family is comprised of 170 species distributed from North to Central America, and at elevations ranging from sea level to nearly 5000 m21,22,23. We assembled a dataset of adult body mass, adult body size (snout-vent length; SVL), thermoregulatory behavior and thermal physiology (field-estimated active and inactive body temperatures, laboratory preferred temperatures, field-measured thermoregulatory effectiveness, and critical thermal limits), metabolic physiology (mass-specific, mass-corrected, and temperature-corrected metabolic rate), and life-history traits (offspring mass, offspring size, clutch/litter size, and annual mass-specific production) by combining newly collected with previously published data from 125 phrynosomatid species (80 oviparous and 45 viviparous species). To assess how phenotypic variation relates to the environments species occupy, we also estimated both broadscale (environmental layers at 1 km2 resolution) and fine-scale (operative environmental temperatures (Te) during activity and inactivity periods) thermal variation for each lizard population. Our dataset includes 73% of phrynosomatids and representatives from all viviparous sub-lineages. We then fitted a series of evolutionary models to the behavioral, physiological, morphological, and reproductive data to determine the patterns of trait evolution associated with oviparity and viviparity, and assess the strength of phenotypic convergence in viviparous species. Through a series of regression approaches, we then investigated the evolutionary relationships between environmental temperatures and parity mode shifts, and how thermoregulatory behavior varies across parity modes and thermal environments.
Here, we show that the evolution of viviparity in phrynosomatids is associated with convergent reductions in cold tolerance, field body temperature, laboratory preferred body temperature, heat tolerance, mass-specific metabolic rate, annual number of offspring, and mass-specific production. Viviparous species maintain behaviorally lower body temperatures of activity (even in warm habitats) and have a lower fecundity than oviparous species, which reduces their energetic burden allocated to maintenance and reproduction. Together, our results indicate that viviparity represents the slow end of the fast-slow life-history continuum and that behavioral and physiological changes associated with viviparity facilitate access to cold habitats. This study sheds new light on the multidimensional patterns of evolution associated with viviparity and, through their interpretation, the factors that underpin its origin in squamates.
Results and discussion
We began by building a phylogeny for phrynosomatids (Supplementary Fig. 1) and reconstructing parity mode across the tree. Our reconstructions strongly support five independent shifts to live-bearing, and no back-transitions to oviparity (Fig. 1a; Supplementary Fig. 2). Transitions from egg laying to live birth in phrynosomatids are strongly associated with an 1.8-fold reduction in the evolutionary optimum (θ parameter; see Methods) for mass-specific metabolic rate during activity (Fig. 1b; Supplementary Table 2). Likewise, across multiple transitions from oviparity to viviparity we detected a 2.4-fold reduction in optimal annual production (Supplementary Table 2). Put together, viviparity in phrynosomatids represents a multidimensional phenotype in which the ratio and quantity of energy allocated to maintenance and production are decreased, a finding robust to repeated origins of live birth (Supplementary Table 6). These evolutionary patterns also align with findings from biophysical approaches, namely that viviparous females have a lower embryonic energy consumption than oviparous females9.
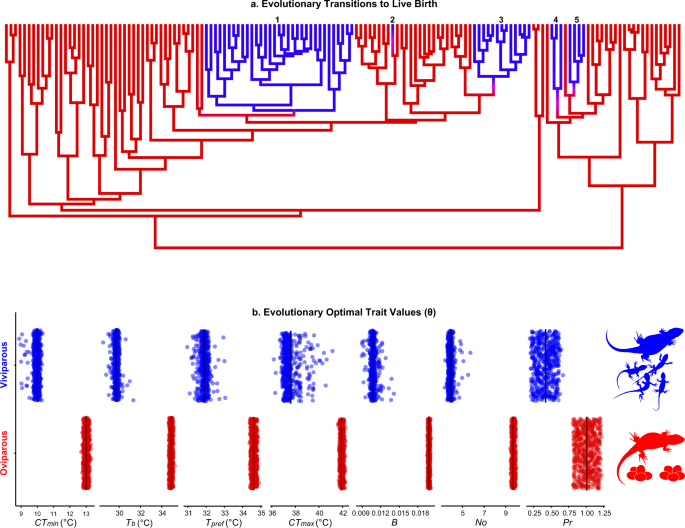
a Five evolutionary transitions from egg laying (red) to live-bearing (blue) occurred in phrynosomatids. b Viviparous lineages are characterized by reductions in the evolutionary optimal trait values (θ) for cold tolerance (CTmin), field-estimated body temperature (Tb), the laboratory-measured preferred body temperature (Tpref), heat tolerance (CTmax), mass-specific metabolic rate during activity (B), the annual number of offspring (No), and mass-specific production (Pr). Evolutionary optimal trait values were inferred from the Ornstein-Uhlenbeck (OU) model-fitting procedure (see Methods). Each point represents a different stochastic character map from our analyses across the maximum clade credibility tree (n = 500 per trait). These patterns are matched with a strong signal of phenotypic convergence among viviparous species (Supplementary Table 6). Source data are provided as a Source Data file.
Our results can be interpreted via a combination of metabolic and life-history theory15,18,19,24. In low-predation environments, populations evolve toward a lower metabolic rate and lower reproductive allotment24. Species with lower mass-specific metabolic rates also exhibit reduced mass-specific production and are positioned on the slow end of the fast-slow life-history continuum15. Given these premises and assuming steady-state populations—populations in which energy invested into production (birth rates) equals energy lost by predation (death rates)19,25—viviparity in phrynosomatids is a high-survivorship, low-fecundity phenotype positioned on the slow end of the fast-slow continuum. This 'slow' life-history strategy is characterized by the reduction in mortality afforded by in utero embryonic development (in comparison to eggs deposited in nests) against abiotic and biotic hazards2,3,4,5,8,26,27,28,29,30,31 and is favored in colder environments such as high elevation8,20,21, where predation risk for phrynosomatids and other ectotherms is lower32,33,34. Furthermore, when compared to their oviparous counterparts, viviparous phrynosomatids are more common in tropical environments with lower temperature seasonality21, where selection could favor allocating energy in fewer, fitter offspring, rather than higher productivity35. Whereas viviparous females can replace themselves each generation by allocating less energy to maintenance and production (normalized by body mass), oviparous females instead expend greater energy on producing more eggs per year (Fig. 1b; Supplementary Table 2).
Pathways for a reduced mass-specific metabolic rate
Shared reductions in mass-specific energetic demand could reflect different evolutionary pathways involving changes in body mass, field body temperature, or both (Table 1a). Across five transitions to viviparity, we do not find any support for adaptive shifts in body mass associated with the reproductive mode in phrynosomatids (Supplementary Table 2). Phylogenetically-corrected body size (SVL), which is strongly correlated with age at sexual maturity12 and with body mass (log10body mass = 0.288log10SVL + 1.522, P < 0.001; Supplementary Table 4), was positively correlated with clutch/litter size (log10clutch or litter size = 1.132log10SVL −1.236, P < 0.001; Fig. 2a; Supplementary Table 4) and offspring size (log10offspring size = 0.289log10SVL + 0.906, P < 0.001; Fig. 2b; Supplementary Table 4). Therefore, whether oviparous or viviparous, larger females are more fecund, and produce larger neonates36,37. Correspondingly, we infer that, during the transition to living birth in phrynosomatids, the evolutionary optimal body size in females38 (θ = 61.1 mm SVL; Supplementary Table 3) likely remains unchanged because any size shift would also affect the quantity and quality of offspring.
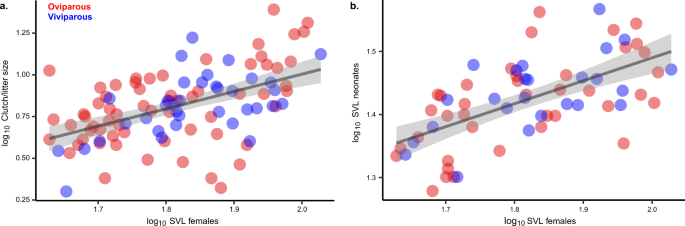
Influence of dam's body size on clutch/litter size (a; y = 1.1321x − 1.2362, two-sided P < 0.001, n = 64 oviparous species and 36 viviparous species) and on the body size of neonates (b; y = 0.2893x + 0.9064, two-sided P < 0.001, n = 39 oviparous species and 25 viviparous species). Data are presented as mean values, and regression lines are wrapped by 95% confidence bands. The regression slopes were estimated by phylogenetic regressions (PGLS), and the source data are provided as a Source Data file.
In contrast to body mass, however, transitions to viviparity are associated with a 5 °C reduction in the optimal field-active body temperatures (θ = 29.7 °C) when compared to their oviparous counterparts (θ = 34.9 °C; Fig. 1b; Supplementary Table 2). Therefore, reductions in core temperature, but not body mass, characterize metabolic changes in the transition to living birth in phrynosomatid lizards (trait combination i in Table 1a). That finding is further supported by our estimated mass-corrected and temperature-corrected metabolic rate: both parity modes share the same optimal temperature-corrected metabolic rate, but viviparity is associated with an 0.9-fold reduction in the optimal mass-corrected metabolic rate of activity (Supplementary Table 2). This finding, based on field-active body temperatures, also holds when considering energetic demand during inactivity: the mass-corrected metabolic rate was lower for viviparous species than their oviparous counterparts (Supplementary Table 2). Given that overly high incubation temperatures negatively affect embryos3,39,40, reductions in the field-active body temperatures of viviparous species could optimize intrauterine embryonic development41. For example, when pregnant Sceloporus jarrovii females are maintained at 36 °C, neonates are smaller and offspring mortality reaches ~50%42. By contrast, in female S. jarrovii that maintain a body temperature of ~32 °C neonates are larger and offspring viability is >95%42. Indeed, during pregnancy (especially late pregnancy) in phrynosomatids body mass and metabolic rate of females increase, and females tend to behaviorally compensate by lowering their body temperature41,43,44,45,46,47,48. For example, a behavioral reduction of 2.5 °C in the preferred temperature of pregnant S. jarrovii likely alters their metabolic rate to that of non-pregnant lizards41.
Lower fecundity reduces mass-specific production in viviparous lizards
In addition to energetic adjustments mediated by thermal behavior, we detected convergent shifts to lower mass-specific production in viviparous species (Supplementary Table 6). Reductions in mass-specific production might reflect different combinations of offspring mass and annual fecundity (Table 1b). Yet, the evolutionary optima for offspring mass (θ = 0.83 g; Supplementary Table 2) and offspring size (θ = 26.4 mm snout-vent-length; Supplementary Table 2) do not vary between viviparous and oviparous species. The retention of optimal offspring size12 and offspring mass in viviparous lineages could reflect the presence of a shared adaptive optimum: indeed, empirical studies on phrynosomatids have found that excessively small or large offspring (based on maternal morphology) typically exhibit reduced survivorship49,50.
As described above, the offspring body size is highly correlated with dam's body size (Fig. 2b), but, crucially, the temperature during embryogenesis also impacts neonate body size39,40,42. Although viviparous species certainly exhibit lower body temperatures when compared to their oviparous relatives, those body temperatures are nonetheless, on average, considerably higher (mean Tb = 31.2 °C, n = 38) than those available in their environments (mean Te = 26.0 °C, n = 28; U = 293.5, two-sided P = 0.002). This finding is consistent with the 'cold-climate hypothesis': females can thermoregulate above ambient temperatures in cold environments to shorten embryonic development time and reduce exposure to overly cold temperatures3,9. Compared with embryos developed in the dam's uterus, eggs in nests in cold habitats are exposed to lower incubation temperatures9. Depending on the temperature, eggs could be viable at low temperatures, but offspring may be smaller, likely related to a longer incubation and consequently high energy consumption9,39,40. Therefore, in cold habitats, viviparity helps maintain the optimal offspring size, whereas oviparity could induce smaller (i.e., poorer quality) neonates39. This effect of intrauterine incubation could represent one of the main reproductive advantages of live-bearing species in cold habitats (consistent with the CCH)3.
Nonetheless, when compared with viviparous species, oviparous phrynosomatids produce ~2.5-fold more offspring per year (Fig. 1b; Supplementary Table 2). The lower annual fecundity and consequent lower mass-specific production in viviparous species could reflect their relatively long gestation periods, which limit most species to a single litter per year16,51. Therefore, the available evidence indicates that in the evolutionary transition to viviparity, selection favors allocating less energy into production, while leaving offspring size unchanged (corresponding to trait combination number iv in Table 1b). As in many other lineages, the differences we observe here among oviparous and viviparous lineages highlight the evolutionary tightrope organisms face between the competing fitness demands of producing high-quality offspring (i.e., high survivorship) and producing more offspring (i.e., high fecundity). For example, a strong association between viviparity and lower fecundity is not only common in squamates but also occurs in gastropods, insects, and fishes, in which lower fecundity is counterbalanced by higher offspring quality and survivorship1,2,52,53.
Patterns and mechanisms of physiological evolution in viviparous lizards
Although viviparous species are conspicuously more prevalent in cooler habitats than oviparous species, they certainly also occur in warm environments20,21. Whether found in relatively cool habitats or in relatively warm habitats (Supplementary Fig. 3), viviparous lizards generally exhibit a lower core temperature than their oviparous counterparts (see Supplementary Data 1). Correspondingly, the field-measured body temperature of viviparous species is poorly correlated with mean annual temperature (Fig. 3b), or with any of our environmental variables (Supplementary Table 4). By contrast, mean annual temperature is positively correlated with body temperature in oviparous lizards (Fig. 3b), although the strength of the relationship is weak (Supplementary Table 5).
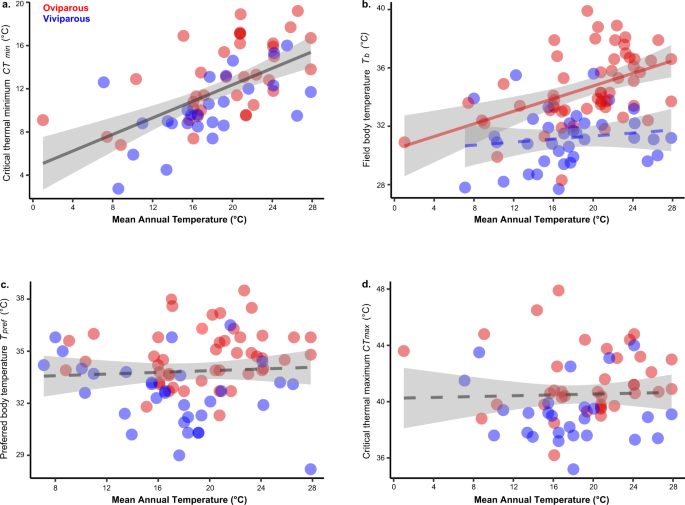
Influence of mean annual temperature (MAT) on cold tolerance (a; y = 0.354x + 5.639, two-sided P < 0.001, n = 36 oviparous species and 26 viviparous species), field body temperature (b; for oviparous: y = 0.230x + 30.3, two-sided P < 0.001, n = 55, and for viviparous: y = 0.055x + 30.69, two-sided P = 0.4, n = 37), preferred body temperature (c; y = −0.0202x + 35.05, two-sided P = 0.6, n = 47 oviparous species and 32 viviparous species), and heat tolerance (d; y = 0.032x + 41.29, two-sided P = 0.5, n = 37 oviparous species and 26 viviparous species). Solid lines represent slopes with statistical significance (<0.05), and dashed lines represent slopes that are not statistically different from 0. Data are presented as mean values, and 95% confidence bands are included around regression lines. The regression slopes were estimated by phylogenetic regressions (PGLS), and the PGLS results using the other macroclimatic predictors (bio10 and bio11) and operative temperatures (Te) are given in Supplementary Table 4. Parity mode did not impact the relationship between MAT and CTmin (a), Tpref (c), or CTmax (d); therefore, data were combined for oviparous and viviparous species (as indicated by a single gray regression line). By contrast, parity mode impacts the relationship between MAT and Tb (b), as the P-value (0.02) was below the Bonferroni-corrected significance level of 0.025. Source data are provided as a Source Data file.
Regardless of parity mode, thermoregulatory effectiveness (i.e., the ability to maintain the core temperature within the preferred range) is uniformly high, and statistically indistinguishable among parity modes in phrynosomatid lizards (U = 467.5, two-sided P = 0.67, n = 37 oviparous species and 27 viviparous species; mean E = 0.8 ± 0.02SE for both modes). Even when viviparous species are found in warm habitats and oviparous species are found in cool habitats, phrynosomatid lizards are effective at maintaining their field body temperature within (or close to) their respective preferred thermal ranges. For example, Sceloporus bulleri, S. macdougalli, S. prezygus, S. serrifer, and S. stejnegeri are viviparous lizards inhabiting relatively warm environments: these species nonetheless maintain a lower preferred body temperature (see Supplementary Data 1). By contrast, Sceloporus aeneus, S. graciosus, S. slevini, and S. vandenburgianus are oviparous lizards from relatively cold environments, and they exhibit a higher preferred body temperature than viviparous counterparts in similar habitats (see Supplementary Data 1).
Furthermore, under the threshold model, we found weak evolutionary covariation between environmental predictors and reproductive mode (mean annual temperature: r = −0.205; the mean temperature of the coldest quarter: r = −0.001; the mean temperature of the warmest quarter: r = −0.359), implying that environmental temperature is not strongly associated with evolutionary transitions to viviparity. Likewise, phylogenetic logistic regressions54 indicate that viviparity is not predicted by mean annual temperature (z = −0.367, P = 0.7, n = 56 oviparous species and 38 viviparous species), mean temperature of the coldest quarter (z = −0.0056, P = 1, n = 56 oviparous species and 38 viviparous species), or mean temperature of the warmer quarter (z = −0.849, P = 0.4, n = 56 oviparous species and 38 viviparous species). Given these results, we infer that cool-adjusted thermal physiology of viviparous species can facilitate access into cooler environments, but that evolution of viviparity need not be a necessary outcome of shifts into cooler environments.
While evolutionary covariation between the thermal environment and reproductive mode is weak, the degree of evolutionary convergence towards a more cool-adjusted thermal physiology in viviparous lizards is remarkably strong (as indicated by the Wheatsheaf index; Supplementary Table 6). The evolutionary optimum for cold tolerance is 3 °C lower in viviparous phrynosomatids (θ = 10.0 °C) than in their oviparous counterparts (θ = 13.0 °C; Fig. 1b; Supplementary Table 2). Likewise, the phenotypic optimum for heat tolerance is 4.3 °C lower in viviparous (θ = 37.6 °C) than oviparous (θ = 41.9 °C) species (Fig. 1b; Supplementary Table 2). In addition to thermal limits, the evolutionary optimum for the preferred body temperature (Tpref) is lower in viviparous species than their oviparous relatives (θ = 31.9 °C for viviparous and 34.6 °C for oviparous species; Fig. 1b; Supplementary Table 2), although the degree of convergence for Tpref was weaker (Supplementary Table 6).
Among these shifts to more cool-adapted physiology in viviparous species, only cold tolerance reflects adjustments to cooler environments. In particular, we found a strong positive relationship between mean annual temperature and cold tolerance in both oviparous and viviparous lineages (Fig. 3a; Supplementary Table 4). This relationship is matched by an instantaneous pace of cold tolerance adaptation (t1/2 = 0 million years, and α = ∞ for both viviparous and oviparous species; Supplementary Table 5). Thus, the reduced cold tolerance of viviparous species likely arises from the fact they are more prevalent in cooler environments than oviparous phyrnosomatids20,21. Enhanced cold tolerance in cooler environments, regardless of parity mode, fits into a broader picture of ecophysiological evolution in ectotherms: cold tolerance is phylogenetically labile55 and often rapidly adapts to the minimum temperatures ectotherms experience in their environments56. By contrast, heat tolerance adapts slowly to the thermal environment itself (Fig. 3d; Supplementary Table 4), in turn reflecting a much longer phylogenetic half-life for this trait (t1/2 = 8.8 million years, and α = 0.08 for viviparous, and t1/2 = 17.8 million years, and α = 0.04 for oviparous species; Supplementary Table 5). Likewise, mean annual temperature is a weak predictor of preferred body temperature both for viviparous and oviparous species (Fig. 3c; Supplementary Table 4), reflecting protracted lags in adaptation (t1/2 = 13.8 million years, and α = 0.05 for viviparous, and t1/2 = 9.7 million years, and α = 0.07 for oviparous species; Supplementary Table 5).
Taken together, our results imply that the thermal behavior and physiological properties of viviparous species are not exclusively by-products of live-bearing species being more common in colder environments. Instead, these patterns are also part and parcel of a broader set of life-history adjustments altering how energy is allocated to growth, maintenance, and reproduction. Given that viviparity and oviparity are on different sides of the 'slow-fast' life-history continuum, transitions to live birth may reflect a number of potential selective trade-offs important for life-history evolution, including (but not limited to) environmental temperature, hypoxia at high elevation, lower food availability, higher intra- and interspecific competition, and high predatory risk in eggs1,2,5,57. In this sense, the 'cold-climate hypothesis' fits into a broader set of energetic trade-offs and selective pressures that may favor the evolution of viviparity. While the inference space of our results is limited to phrynosomatid lizards, the general principles that explain evolutionary patterns in this system also characterize other ectotherm lineages, which account for nearly all origins of viviparity in animals. We suspect, therefore, that the patterns we observed here might be generalizable across the animal tree of life.
Methods
Ethics statement
The data collection and experiments were conducted in accordance with the collecting permits (SGPA/DGVS/07946/08, 03369/12, 00228/13, 07587/13, 01629/16, 01205/17, 02490/17, 06768/17, 000998/18, 002463/18, 002490/18, 002491/18, 003209/18, and 02523/19) approved by Dirección General de Vida Silvestre, México.
Phylogeny and divergence time estimation
To estimate the phylogeny and divergence time among phrynosomatid species we used sequences of five mitochondrial and eight nuclear genes available in GenBank for 149 taxa (Supplementary Data 2). Accession numbers were the same as those used in Martínez-Méndez et al.58 for the Sceloporus torquatus, S. poinsettii and S. megalepidurus groups and the same as those in Wiens et al.59 for other phrynosomatid species. For taxa not included in the previous references, we searched GenBank for available sequences. We then performed alignments for each gene using MAFFT (ver. 7)60 and concatenation and manual refinement using Mesquite (ver. 3.6);61 obtaining a concatenated matrix of 9837 bp for 149 taxa (Supplementary Data 3). For the relaxed clock analyses, three nodes were calibrated using lognormal distributions based on two previous studies59,62. The first calibration was set for the Sceloporus clade (offset 15.97 million years ago (MYA)) based on a fossil Sceloporus specimen63). The second calibration point was set for the Phrynosoma clade (offset 33.3 MYA) based on the fossil Paraphrynosoma greeni64, and the last calibration point was for the Holbrookia-Cophosaurus stem group (offset 15.97 MYA) given the fossil Holbrookia antiqua63. We conducted dating analysis with the concatenated sequences matrix, partitioned the mitochondrial and nuclear information, each gene under GTR + I + Γ model, and allowed independent parameter estimation. We performed Bayesian age estimation with the uncorrelated lognormal relaxed clock (UCLN) model in BEAST (ver. 2.5.2)65,66 and run on CIPRES67. Tree prior (evolutionary model) was under the Birth-Death model, and we ran two MCMC analyses for 100 million generations each and stored every 20,000 generations. We assessed the convergence and stationarity of chains from the posterior distribution using Tracer (ver. 1.7)68. We combined independent runs using LogCombiner (ver. 2.5.2; BEAST distribution)69 and discarded 30% of samples as burn-in, obtaining values of effective sample size (ESS) greater than 200. We estimated the maximum clade credibility tree from all post-burnin trees using TreeAnnotator (ver. 1.8.4)69. The ultrametric tree is available as Supplementary Data 4. As we describe below, we accounted for phylogenetic uncertainty in our models by reperforming analyses using 500 trees that we randomly sampled from our posterior distribution. The 500 sampled trees are available as Supplementary Data 5.
Data collection
Parity mode
We categorized each species as either oviparous or viviparous based on previously published databases21,37,51,70, published references, and unpublished data (Supplementary Data 1). Our assignations align with other studies, except for one species, Sceloporus goldmani, which has been previously considered a viviparous species21,71,72,73. The only available sequence in GenBank (U88290) for that species is from a male (MZFC-05458) collected in Coahuila, Mexico72. However, in that same locality, one of us (F. R. Méndez-de la Cruz; unpubl. data) collected two females of the same species, and both laid eggs. Thus, the population of S. goldmani herein included is considered oviparous. Considering S. goldmani viviparous increases the number of originations of viviparity to 6 (from 5) in this lineage (Supplementary Fig. 4), but does not alter the outcome of our model-fitting analyses of trait evolution (Supplementary Table 7).
Thermal physiology
We compiled a database of four thermal physiological traits that influence the performance and fitness of ectotherms74 for 104 phrynosomatid species. These data were gathered from both published sources and from our own field and laboratory work (Supplementary Data 1). The thermal physiological traits we examined were the field body temperature (Tb) of active lizards, the preferred body temperature (Tpref) in a laboratory thermal gradient75, cold tolerance (critical thermal minimum, CTmin), and heat tolerance (critical thermal maximum, CTmax). These latter two traits (CTmin and CTmax) describe the thermal limits of locomotion; specifically, they describe the lower and upper temperatures, respectively, at which lizards fail to right themselves when flipped onto their backs55,76. To minimize the confounding effects of experimental design, we limited our data selection to species that were measured with similar methods. Correspondingly, our new data collection approach mirrored that of the published studies from which we extracted data. To obtain mean values for each thermal physiological trait (CTmin, Tb, Tpref, and CTmax) we did not mix data measured from different locations (instead, we used data from the population with the highest sample size).
For species that we newly measured thermal physiological traits, we obtained the data as we describe below, and we based our methodology on the previous work55,56,75,76. We captured active (perching) adult lizards by lasso or by hand, and immediately (<10 s) we measured their body (cloacal) temperature using a thermocouple (type K) connected to a digital quick-reading thermometer. We transported lizards to a field laboratory (which was at an ambient temperature of ~20 °C), measured the SVL and body mass of individuals, and the next day we measured their preferred body temperature by placing them into a laboratory thermal gradient from 8:00 to 17:00 h. The laboratory thermal gradient consisted of a wooden box (100 cm wide, 100 cm long, and 30 cm tall) divided into ten tracks. At one extreme of the laboratory thermal gradient, we placed ten 75 W bulbs (one per track) at the height of 25 cm above to generate a thermal gradient ranging from ~50 °C in the hot extreme to ~20 °C in the cold extreme. Then, we placed each lizard on a track, and we measured their cloacal temperature (using the same thermocouple and thermometer as in the fieldwork) every hour, during the length of the experiment. When we finished the experiment on thermal preferences, we performed the experiment on heat tolerance. For that, we placed individually each lizard into a plastic container (25 cm diameter and 30 cm height) and we increased their body temperature (1 °C/m) using a 90 W bulb suspended ~40 cm above the container. When lizards initiated panting behavior, we began flipping them onto their back every 20 s, and we recorded CTmax as the body temperature at which a lizard lost the ability to right itself. On the next day, we each placed individually each lizard into a plastic container (23 cm wide, 16 cm long, and 8 cm tall), and the plastic container on a bed of ice. Every 20 s we began flipping lizards onto their back, and we recorded CTmin as the body temperature at which a lizard lost the ability to right itself. For CTmax, and CTmin experiments, we did not include pregnant/gravid females, and after laboratory experiments, we hydrated lizards ad libitum, and released them at their capture sites.
In Supplementary data 1 we indicate coordinates where thermal physiological traits were measured and the environmental variables associated with each coordinate. In cases where locality details, but not coordinates, were available, we georeferenced sampling sites using Google Earth Pro (Version 7.3.3). All physiological data correspond only to adult lizards. Some studies have found that pregnant females reduce their core temperature to better match the optimal incubation temperature for their offspring41,43,48. When we detected effects of reproductive condition on Tb or Tpref, we excluded data from pregnant (or gravid) females. To test whether behavioral and physiological traits differed between sexes, we performed t-tests for a sub-set of 25 species (Supplementary Table 1). We did not find significant behavioral and thermal physiological differences between (non-gravid/non-pregnant) females and males in Tb (t = 0.172, df = 48, P = 0.86), Tpref (t = −0.482, df = 48, P = 0.63), CTmin (t = 0.742, df = 45, P = 0.46), or CTmax (t = −0.407, df = 42, P = 0.69), so we combined data for both sexes. Ideally, we would rerun all analyses using thermal trait data from gravid/pregnant females, but such data are still lacking. Given that, in the few cases where robust data do exist, preferred temperatures in pregnant females tend to be even lower than in non-pregnant females46,47,77, we suspect that our analyses provide a relatively conservative estimate of physiological differences among parity modes.
Operative temperatures
As we describe below, we were interested in estimating thermoregulatory patterns among phrynosomatid species. Doing so requires knowledge of the environmental operative temperatures (Te) available to lizards. Te represents the equilibrium temperature of an animal in the absence of behavioral thermoregulation78. We recorded Te using previously-calibrated pipe models (made of polyvinylchloride), which were similar in shape, size, and heat gain/loss with respect to lizards of each species (for examples of calibration, see refs. 56,78,79). Into each pipe model, we inserted one temperature data logger (Thermochron iButton; model DS1921G), which recorded temperature (±0.1 °C) every ten minutes during the same periods (and days) during which we were measuring field-active body temperatures (Tb) in lizards. These models were placed randomly in microsites occupied by lizards in their activity period56,79. Operative temperatures were typically measured during a sampling period of 1–5 days for each locality, which always occurred during times of the year when lizards exhibit surface activity. The pipe models also recorded temperature during the night (Te-night), which we used as an approximation of core temperatures of individuals during their inactivity period to model mass-corrected metabolic rates during inactivity (described below). As a caveat, the Te-night measured in microsites where individuals were active are likely to be somewhat cooler than microenvironmental temperatures experienced by inactive lizards in their retreats.
Thermoregulatory effectiveness
Several studies have found that viviparous species have lower field body temperatures than their oviparous counterparts11,80. Less well known, however, is whether lower field body temperatures reflect a behaviorally passive property of viviparous lizards, perhaps because of their distributions in relatively cooler habitats, or whether those low field body temperatures reflect a more behaviorally active decision to thermoregulate. Therefore, we were particularly interested in the thermoregulatory patterns of oviparous and viviparous species. We calculated the effectiveness of temperature regulation (E), a ratio that describes how well lizards maintain their Tb within their Tset range (central 50% of data of Tpref; Tset25, and Tset75), given the operative temperatures (Te) available in their habitat75. We estimated E for each species following the equation proposed by Hertz et al.75:
where \(\overline{{db}}\) is the average of the accuracy of body temperature, and indicates the deviation of Tb from Tset range. If each Tb < Tset25, then each db = Tset25 − Tb, if each Tb > Tset75, then each db = Tb − Tset75, and if each Tb is within Tset range, then each db = 0. \(\overline{{de}}\) is the average of thermal quality of the habitat, and indicates the deviation of Te from Tset range. If each Te < T<...

Comments
Post a Comment